...
| Excerpt |
|---|
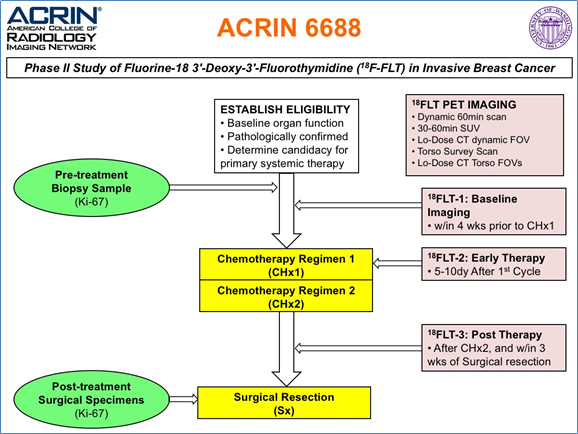
The objective of the ACRIN 6688 multi-center clinical trial was to correlate changes measured by 18F-FLT PET imaging, a measure of cellular proliferation, in the primary tumor early during NAC (neo-adjuvant chemotherapy) with pCR (pathologic complete response) in locally advanced breast cancer patients. The trial also examined both pre-therapy and post-therapy association of 18F-FLT uptake with the tissue proliferative marker Ki-67 to compare 18F-FLT PET/CT against an accepted reference standard for cellular proliferation. The trial protocol is graphically described in the figure below, and appears online in the trial protocol https://www.acrin.org/Portals/0/Protocols/6688/Protocol-ACRIN_6688-Amend9_ v092011_WEB.pdf: Potentially three 18 FLT/CT imaging sessions would be conducted at the times indicated above for the 90 enrolled patients on the study. However, only 43 patients completed all three scans, 54 patients had pre/post therapy scans of which 51 were evaluable for the primary aim (see Kostakaglu et al. 2015 ). Acknowledgements This shared data set was provided by Paul R. Jolles, MD, and David Mankoff, MD, PhD, and Lale Kostakoglu, MD, MPH, in collaboration with the American College of Radiology Imaging network (ACRIN)Core Lab. Many thanks are due to the ACRIN 6688 trial team, and all the patients participating in the study. This study was supported by American College of Radiology Imaging network (ACRIN), which received funding from the National Cancer Institute through UO1 CA080098, U01 CA190254 and R50 CA211270 (Muzi), under the American Recovery and Reinvestment ACT of 2009 (ARRA) and UO1 CA079778. Please see QIN ECOG-ACRIN Data Sharing page for an overview and list of other ECOG-ACRIN data collections available on TCIA. |
...