| ACRIN | American College of Radiology Imaging Network |
| I-SPY TRIAL | Investigation of Serial Studies to Predict Your Therapeutic Response with Imaging and moLecular Analysis |
| CALGB | Cancer and Leukemia Group B |
| TCIA | The Cancer Imaging Archive |
| NACT | Neoadjuvant chemotherapy |
| SER | Signal Enhancement Ratio |
| FCM | Functional Tumor Volume |
ACRIN 6657 was designed as a prospective study to test MRI for ability to predict response to treatment and risk-of-recurrence in patients with stage 2 or 3 breast cancer receiving neoadjuvant chemotherapy (NACT). ACRIN 6657 was conducted as a companion study to CALGB 150007, a correlative science study evaluating tissue-based biomarkers in the setting of neoadjuvant treatment of breast cancer. Collectively, CALGB 150007 and ACRIN 6657 formed the basis of the multicenter Investigation of Serial Studies to Predict Your Therapeutic Response with Imaging and moLecular Analysis (I-SPY TRIAL) breast cancer trial, a study of imaging and tissue-based biomarkers for predicting response and survivalAdd protocol references for all three studies.
Participant Eligibility and Enrollment: Criteria for inclusion were patients enrolling on CALGB 150007 with T3 tumors measuring at least 3 cm in diameter by clinical exam or imaging and receiving neoadjuvant chemotherapy with an anthracycline-cyclophosphamide regimen alone or followed by a taxane. Pregnant patients and those with ferromagnetic prostheses were excluded from the study. The study was open to enrollment from May 2002 to March 2006. 237 patients were enrolled, of which 230 met eligibility criteria.
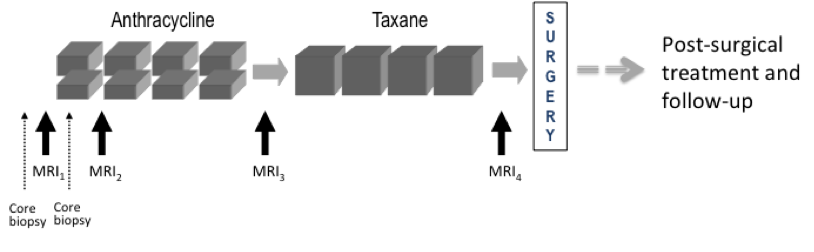
Imaging time points: MRI exams were performed within four weeks prior to starting anthracycline-cyclophosphamide chemotherapy (T1, MRI1), at least 2 weeks after the first cycle of AC and prior to the second cycle of AC (T2, MRI2), between anthracycline-cyclophosphamide treatment and taxane therapy if taxane was administered (T3, MRI3), and after the final chemotherapy treatment and prior to surgery (T4, MRI4). The study schema is shown in Figure 1

Figure 1. CALGB 150007 and ACRIN 6657 study schema.
Imaging protocol: MR imaging was performed on a 1.5 Tesla field strength scanner using a dedicated breast radiofrequency coil. The image acquisition protocol included a localization scan and T2-weighted sequence followed by a contrast-enhanced T1-weighted series. All imaging was performed unilaterally over the symptomatic breast and in the sagittal orientation. The contrast-enhanced series consisted of a high resolution (≤1mm in-plane spatial resolution) three-dimensional, fat-suppressed, T1-weighted gradient echo sequence with TR≤20 ms, TE = 4.5 ms, flip angle ≤ 45º, 16-18 cm field-of-view, minimum matrix 256x192, 64 slices, slice thickness ≤ 2.5 mm. Scan time length for the T1-weighted sequence was required to be between 4.5 and 5 minutes. The sequence was acquired once before contrast injection and repeated at least twice following injection.
Tumor diameter measurement and volumetric analysis: Tumor longest diameter (LD) was measured by the site radiologist as the greatest extent of disease on baseline MR images, including intervening areas of non-enhancing tissue. The same measurement direction was used on all subsequent MRI exams. The primary predictor variable, functional tumor volume (FTV) was measured from contrast-enhanced images using the signal enhancement ratio (SER) method. Voluetric analysis, including Quality Control assessment, was performed centrally at the breast MR imaging laboratory at University of California at San Francisco (UCSF).
This is a limited access data set. To request access, please submit a concept sheet ((((link to ACRIN doc.))) to (ACRIN Contact). If access is granted you will be able to view and download these images on The Cancer Imaging Archive (TCIA) by logging in and selecting the I-SPY1 collection. This data set will be made publicly available on or before XXXX XX, 20XX. |
Collection Statistics | (updated 6/18/2015) |
|---|---|
Modalities | MR |
Number of Patients | 222 |
Number of Studies | 847 |
Number of Series | 7880 |
Number of Images | 386,528 |
| Images Size (GB) | 76.2 GigaBytes |
Acknowledgements
This shared data set was provided by the Breast Imaging Research Program at UCSF, in collaboration with ACRIN, CALGB, the I-SPY TRIAL, and TCIA.
David Newitt, PhD, UCSF, Nola Hylton, PhD, UCSF
| UCSF | NIH R01 CA132870 and U01 CA151235 |
| ACRIN | NIH UO1 CA079778 and UO1 CA080098 |
| CALGB | NIH UO1 CA31964 and UO1 CA33601 |
((( Insert full acknowledgement lists here )))
If you are unsure how to download this Collection please view Searching by Collection or refer to The Cancer Imaging Archive User's Guide for more detailed instructions on using the site.
The processing of the MR image data for ACRIN 6657 consisted of the following steps between image acquisition and the creation of this shared data set on TCIA:
While every effort was made to preserve the integrity of both the original image data and image meta-data (DICOM attributes, public and private), multiple file transfers and strict adherence to HIPPA guidelines for patient confidentiality may have resulted in loss of some data. If any questions arise, or patient PHI is found in any data on this collection, please contact David Newitt at the Breast Imaging Research Program, UCSF (david.newitt@ucsf.edu).
In addition to the complete set of ACRIN 6657 imaging studies, curated data sets based on UCSF QC assessment, protocol compliance and data completeness are provided for download in the form of TCIA shared lists. These image data sets are accompanied by Excel files with patient clinical and outcome data.
847 MR on-study studies (222 subjects) in UCSF image database.
One patient in the image data collection (I-SPY ID 1079 ) does not appear in the Feb. 2, 2011 I-SPY FINAL LOCKED clinical data set. So no clinical data is available.
Studies with MRI measured longest diameter
839 MR studies have LD reported in the I-SPY 1 clinical database
834 MR studies (219 subjects) in UCSF image database with LD
5 studies that have LD measurement are missing from | ||
|---|---|---|
1071, T1 | 1101, T3 | 1187, T4 |
Level 2a consists of 708 MR studies (208 subjects) in the UCSF image database which, following quality review in 2014, were judged to have sufficiently good image quality and protocol compliance for volumetric DCE SER analysis. Rejection criteria included: incomplete volumetric DCE acquisitions, lack of a 2nd post-contrast acquisition, variability in fat suppression across the image, observed patient motion during the DCE acquisition
7 studies in Level 2a that do NOT have LD measures: | ||
|---|---|---|
ID 1059, T4 | ID 1192, T2 | ID 1215, T1 |
This data is comprised of the patient studies analyzed and reported to ACRIN in 2008, and used for the 2012 Radiology paper on ACRIN 6657 *. This data set is not provided as a shared list, as it is not recommended for use in further analysis. It is described here because it is the data set from which the Level 3 (primary aim analysis) set was derived.
Level 2b consists of 707 MR studies (207 subjects) in the UCSF image database which were submitted to ACRIN at the close of the trial in 2008. Inclusion and exclusion was determined by quality and protocol reviews available at that time. In addition to the exclusion criteria listed for Level 2a, studies done with imaging in the axial plane, in violation of the sagittal orientation specified in the trial imaging protocol, were excluded due to processing limitations of the analysis software. Similarly, bi-lateral sagittal acquisitions (alternating left and right volumetric acquisitions) were excluded.
15 studies accepted for SER analysis since 2008 (in Level 2a but not in 2b) | ||
|---|---|---|
ID 1005, T3 | ID 1110, T4 | ID 1203, T4 |
14 studies rejected since 2008 (in Level 2b but not in 2a) | ||
|---|---|---|
ID 1035, T4 * | ID 1055, T1 * | ID 1206, T1 |
* Subjects that were included in the primary aim analysis (Level 3) | ||
586 MR studies (162 subjects) in UCSF image database
| 45 subjects excluded from Level 2b set | |||||
|---|---|---|---|---|---|
ID 1027 ID 1040 ID 1045 ID 1046 ID 1048 ID 1054 ID 1063 ID 1067 | ID 1079 ID 1084 ID 1103 ID 1110 ID 1120 ID 1137 ID 1139 ID 1152 | ID 1157 ID 1159 ID 1160 ID 1167 ID 1171 ID 1176 ID 1177 ID 1180 | ID 1182 ID 1185 ID 1187 ID 1189 ID 1192 ID 1194 ID 1203 ID 1206 | ID 1210 ID 1212 ID 1214 ID 1215 ID 1219 ID 1221 ID 1222 ID 1228 | ID 1234 ID 1235 ID 1237 ID 1238
ineligible: Case #: 128 |
To download image data sets for the curated data sets go to Tools / Search Shared Lists after logging in TCIA, and search for "ISPY1"
Shared lists have been created for each of the main curated data sets: Level 1, 2a, and 3. For each level one may download either:
"All Series" full MRI datasets including all original series and derived PE, SER maps and segmentation objects from the primary analysis
"DCE + Derived" Original DCE series and derived PE, SER maps and segmentation objects from the primary analysis
"DCE Only" Original DCE series only
Relative sizes of the lists and full collection are shown in the Table below. Estimated download times can be found by loading the shared list into the download manager from the Search Shared Lists tool. Links are also provided in the Table for Excel spreadsheet files containing selected patient clinical and outcome data for the patients in each set. Further data will be made available in the future as it is released by I-SPY. Outcome data may not be accessible by all users.
subjects N | All Series N / Size | DCE + Derived N / Size | N DCE Only N / Size | Clinical data | Outcome data | |
|---|---|---|---|---|---|---|
| Level 0: Complete image data set | 222 | 7880 / 76GB | NA | NA | ||
| Level 1: Studies with MRI LD measurements | 219 | 7778 / 75GB | NA | NA | ||
| Level 2a: Studies with SER Volume measurements | 208 | 6971 / 63GB | 4022 / 43GB | 1243 / 24GB | ||
| Level 3: Studies used in primary aim analysis | 162 | 5696 / 49GB | 3223 / 34GB | 961 / 18GB |
To download: Tools / Search Shared Lists
A data dictionary is provided. The dictionary includes descriptions of all private DICOM attributes used in the derived maps and segmentations generated by the volumetric analysis and QC evaluations. Also included are descriptions of ancillary data provided, including demographic, clinical and survival data.