Summary
BACKGROUND
ACRIN 6657 was designed as a prospective study to test MRI for ability to predict response to treatment and risk-of-recurrence in patients with stage 2 or 3 breast cancer receiving NACT. ACRIN 6657 was conducted as a companion study to CALGB 150007, a correlative science study evaluating tissue-based biomarkers in the setting of neoadjuvant treatment of breast cancer. Collectively, CALGB 150007 and ACRIN 6657 formed the basis of the multicenter Investigation of Serial Studies to Predict Your Therapeutic Response with Imaging and moLecular Analysis (I-SPY TRIAL) breast cancer trial, a study of imaging and tissue-based biomarkers for predicting response and survivalAdd protocol references for all three studies. DCN: Added links above, but not sure they are the best ones.
Participant Eligibility and Enrollment: Criteria for inclusion were patients enrolling on CALGB 150007 with T3 tumors measuring at least 3 cm in diameter by clinical exam or imaging and receiving neoadjuvant chemotherapy with an anthracycline-cyclophosphamide regimen alone or followed by a taxane. Pregnant patients and those with ferromagnetic prostheses were excluded from the study. The study was open to enrollment from May 2002 to March 2006. 237 patients were enrolled, of which 230 met eligibility criteria.
Requirements for MR imaging (As specified in the ACRIN 6657 protocol )
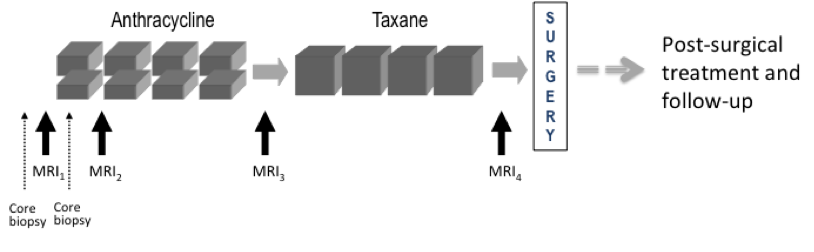
Imaging time points: MRI exams were performed within four weeks prior to starting anthracycline-cyclophosphamide chemotherapy (MRI1), at least 2 weeks after the first cycle of AC and prior to the second cycle of AC (MRI2), between anthracycline-cyclophosphamide treatment and taxane therapy if taxane was administered (MRI3), and after the final chemotherapy treatment and prior to surgery (MRI4). The study schema is shown in Figure 1
Figure 1. I-SPY 1 TRIAL and ACRIN 6657 study schema.
Imaging protocol: MR imaging was performed on a 1.5 Tesla field strength scanner using a dedicated breast radiofrequency coil. The image acquisition protocol included a localization scan and T2-weighted sequence followed by a contrast-enhanced T1-weighted series. All imaging was performed unilaterally over the symptomatic breast and in the sagittal orientation. The contrast-enhanced series consisted of a high resolution (≤1mm in-plane spatial resolution) three-dimensional, fat-suppressed, T1-weighted gradient echo sequence with TR≤20 ms, TE = 4.5 ms, flip angle ≤ 45º, 16-18 cm field-of-view, minimum matrix 256x192, 64 slices, slice thickness ≤ 2.5 mm. Scan time length for the T1-weighted sequence was required to be between 4.5 and 5 minutes. The sequence was acquired once before contrast injection and repeated at least twice following injection.
Tumor diameter measurement and volumetric analysis: Tumor longest diameter (LD) was measured by the site radiologist as the greatest extent of disease on baseline MR images, including intervening areas of non-enhancing tissue. The same measurement direction was used on all subsequent MRI exams. The primary predictor variable, functional tumor volume (FTV) was measured from contrast-enhanced images using the signal enhancement ratio (SER) method. Voluetric analysis, including Quality Control assessment, was performed centrally at the breast MR imaging laboratory at University of California at San Francisco (UCSF).
LINKS
- ACRIN 6657 Protocol http://www.acrin.org/6657_protocol.aspx
- I-SPY TRIAL http://ncicb.nci.nih.gov/tools/translation_research/isp
- CALGB 150007 http://www.cancer.gov/clinicaltrials/search/view?cdrid=69280&version=HealthProfessional
- Curated Data Sets Description
- Data Dictionary
Abbreviations
ACRIN American College of Radiology Imaging Network
I-SPY TRIAL Investigation of Serial Studies to Predict Your Therapeutic Response with Imaging and moLecular Analysis
CALGB Cancer and Leukemia Group B
NACT Neoadjuvant chemotherapy
FTV Functional Tumor Volume
Data Access
Imaging Data
This is a limited access data set. To request access, please contact (ACRIN Contact) help@cancerimagingarchive.net. If access is granted you will be able to view and download these images on The Cancer Imaging Archive (TCIA) by logging in and selecting the I-SPY1 collection. This data set will be made publicly available on or before June 1, 2016.
Collection Statistics | (updated 11/21/2014) |
|---|---|
Modalities | MR |
Number of Patients | 222 |
Number of Studies | 847 |
Number of Series |
|
Number of Images |
|
| Images Size (GB) |
If you are unsure how to download this Collection please view our quick guide on Searching by Collection or refer to our The Cancer Imaging Archive User's Guide for more detailed instructions on using the site.
Shared Lists
- Coming soon
Imaging Data Transfer Process
- De-identified image data were centrally archived at ACRIN Core Lab
- Archived data was sent to the breast MR imaging laboratory at University of California at San Francisco (UCSF) for volumetric analysis.
- De-identified image data, derived analysis maps and segmentations, and ancillary data files were transferred from UCSF to TCIA for data sharing.
Summary of Curated Data Sets
In addition to the complete set of ACRIN 6657 imaging studies, curated data sets based on UCSF QC assessment and data completeness are provided in the form of TCIA shared lists for selective downloads. These data sets are described in the Curated Data Sets Description.
Level 0: All I-SPY 1 Dataset
847 MR on-study studies (222 subjects) in UCSF image database.
One patient in the image data collection (I-SPY ID 1079 ) does not appear in the Feb. 2, 2011 I-SPY FINAL LOCKED clinical data set. So no clinical data is available.
Level 1: LD Dataset
Studies with MRI measured longest diameter
839 MR studies have LD reported in the I-SPY 1 clinical database
834 MR studies (219 subjects) in UCSF image database with LD
5 studies that have LD measurement are missing from the UCSF and ACRIN TRIAD image data collections: |
|
|
|
1071, T0 | 1101, T2 | 1187, T3 |
Level 2a: Good SER Volume Dataset – updated 9/3/14
708 MR studies (208 subjects) in UCSF image database
7 studies in Level 2a that do NOT have LD measures: |
|
|
|
ID 1059, T3 | ID 1192, T1 | ID 1215, T0 | |
|
|
|
|
Level 2b: SER Volume Dataset submitted to ACRIN in 2008
707 MR studies (207 subjects) in UCSF image database
Differences between Levels 2a and 2b:
15 exams accepted for SER analysis since 2008 (in Level 2a but not in 2b) |
|
|
|
ID 1005, T2 | ID 1110, T3 | ID 1203, T3 |
14 exams rejected since 2008 (in Level 2b but not in 2a) |
|
|
|
ID 1035, T3 | ID 1055, T0 | ID 1206, T0 |
Data Dictionary
A data dictionary is provided. The dictionary includes descriptions of all private DICOM attributes used in the derived maps and segmentations generated by the volumetric analysis and QC evaluations. Also included are descriptions of ancillary data provided, including demographic, clinical and survival data.