Summary
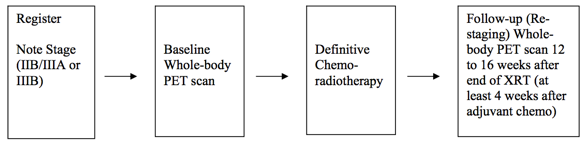
Positron Emission Tomography Pre- and Post-treatment Assessment for Locally Advanced Non-small Cell Lung Carcinoma This was a multicenter clinical trial by the ACRIN Cooperative Group (now part of ECOG-ACRIN) and the RTOG Cooperative Group (now part of NRG) using FDG-PET imaging both pre- and post-chemoradiotherapy. The objective of the ACRIN 6668 multi-center clinical trial was to determine if the PET standardized uptake value (SUV) measurement from FDG-PET imaging shortly after treatment is a useful predictor of long-term clinical outcome (survival) after definitive chemoradiotherapy. Eligible patients were those older than 18 years with AJCC-criteria clinical stage IIB/III non-small cell lung carcinoma who were being planned for definitive concurrent chemoradiotherapy (inoperable disease). Primary Aim Findings: Higher post-treatment tumor SUV (SUVpeak, SUVmax) is associated with worse survival in stage III NSCLC, although a clear SUV cutoff value for routine clinical use as a prognostic factor was uncertain [1]. Later analyses found that larger pre-treatment metabolic tumor volumes (MTVs) were associated with significantly worse overall survival [2]. Other secondary analyses found potentially predictive image texture biomarkers. Study Design Summary: Patients received conventional concurrent platinum-based chemoradiotherapy without surgery; post-radiotherapy consolidation chemotherapy was allowed. A baseline whole-body FDG-PET scan was performed prior to therapy. A second post-treatment whole-body FDG-PET scan occurred approximately 14 weeks after radiotherapy (at least 4 weeks after adjuvant chemotherapy). Pre-treatment FDG-PET scans were performed on ACRIN-qualified scanners. Post-treatment FDG-PET scans were required to be performed within 12–16 weeks after completion of therapy, using the same scanner as that used for the pre-treatment scans. Acknowledgements This shared data set was provided in collaboration with the American College of Radiology Core Lab. Many thanks are due to the ACRIN 6668 trial team, and all the patients participating in the study. This study was supported by the ACRIN Cooperative Group (now part of ECOG-ACRIN) and the RTOG Cooperative Group (now part of NRG) which received funding from the National Cancer Institute through UO1 CA080098 and UO1 CA079778.
Data Access
This is a limited access data set and is only available to members of NCI's Quantitative Imaging Network (QIN). If you are a member of the QIN and would like to request access, please submit a CCP proposal to the QIN Coordinating Committee. Once access is granted, click the Download button to save a ".tcia" manifest file to your computer, which you must open with the NBIA Data Retriever. Click the Search button to open our Data Portal, where you can browse the data collection and/or download a subset of its contents.
Click the Versions tab for more info about data releases.
Detailed Description
Collection Statistics |
|
|---|---|
Modalities | PT, CT, MR, CR, DX, SC |
Number of Patients | 193 |
Number of Studies | 828 |
Number of Series | 2,981 |
Number of Images | 436,165 |
| Image Size (GB) | 127.8 |
Study Accrual: Accrual began in June 2005 and ended in May 2009. Thirty-seven institutions accrued 250 patients to the study. Sixteen patients were ineligible, and eight patients did not have evaluable pretreatment PET, leaving 226 patients. Of these 226 patients, 173 had evaluable post- treatment PET, representing the analysis cohort for the primary end point.
Imaging Protocol: Conventional modern equipment/techniques for FDG-PET (with or without PET/CT) were used in this study. Patients had to fast for 4 hours and have a blood glucose level less than 200 mg/dL before FDG injection. The FDG dose was not mandated; the recommended dose was 0.14 to 0.21 mCi/kg (approximately 10 to 20 mCi). Emission scanning began 50 to 70 minutes after FDG injection and included the body from upper/mid neck to proximal femurs. Acquisition times for emission and transmission scans were in accordance with the manufacturer’s recommendations.
Image Analysis: PET scans were interpreted qualitatively and quantitatively by nuclear medicine physicians/radiologists at each institution, using standardized reporting forms to record the FDG uptake in the primary tumor, regional lymph nodes, and common sites of distant metastasis (ie, bones, adrenals, liver, contralateral lung). These local reviewers were provided with educational materials on image interpretation, specifically describing how to measure peak SUV (SUVpeak). However, formal demonstration of expertise was not mandated. SUVs for regions of interest (ROIs) were determined using two different metrics, maximum SUV (SUVmax) and SUVpeak. SUVmax represented the highest single-voxel SUV within the ROI. SUVpeak represented the mean SUV within a small circular ROI (0.75 to 1.5 cm in diameter) that encompasses the SUVmax voxel. (Thus, SUVpeak will always be lower than SUVmax.).
In addition to the institutional interpretations, pre- and post-treatment PET scans were centrally reviewed at ACRIN by an expert nuclear medicine physician with extensive experience in FDG-PET. A single dedicated workstation was used for this purpose, and SUVpeak was measured with an automated program in a circular ROI 1.5 cm in diameter. The central reader was blinded to clinical data and the institutional SUV measurements.
Outcomes: Patients were observed for a minimum of 2 years (or until death) after completion of treatment in accordance with standard clinical practice. Non-protocol PET imaging was allowed but not mandated.
Schema
Metadata
Much more information about this data set (ACRIN 6668 / RTOG 0235) can be found https://www.acrin.org/6668_protocol.aspx
Citations & Data Usage Policy
This is a limited access data set and is only available to members of NCI's Quantitative Imaging Network (QIN) until 12/11/2018. If you are a member of the QIN and would like to request access, please submit a CCP proposal to the QIN Coordinating Committee. Upon receiving access you may only use it for the purposes outlined in your proposal. You are not allowed to redistribute the data or use it for other purposes. See TCIA's Data Usage Policies and Restrictions for additional details. Questions may be directed to help@cancerimagingarchive.net.
Please be sure to include the following citations in your work if you use this data set:
Data Citation
(authors). (2018). Data from . The Cancer Imaging Archive. http://doi.org (Coming soon)
Publication Citation
Machtay M, Duan F, Siegel BA, et al. Prediction of survival by [18F]fluorodeoxyglucose positron emission tomography in patients with locally advanced non-small-cell lung cancer undergoing definitive chemoradiation therapy: results of the ACRIN 6668/RTOG 0235 trial. J Clin Oncol. 2013;31:3823-3830. http://dx.doi.org/10.1200/jco.2012.47.5947.
Bazan JG, Duan F, Snyder BS, et al. Metabolic tumor volume predicts overall survival and local control in patients with stage III non-small cell lung cancer treated in ACRIN 6668/RTOG 0235. Eur J Nucl Med Molec Imaging. 2017;44:17-24. http://dx.doi.org/10.1007/s00259-016-3520-4.
TCIA Citation
Clark K, Vendt B, Smith K, Freymann J, Kirby J, Koppel P, Moore S, Phillips S, Maffitt D, Pringle M, Tarbox L, Prior F. The Cancer Imaging Archive (TCIA): Maintaining and Operating a Public Information Repository, Journal of Digital Imaging, Volume 26, Number 6, December, 2013, pp 1045-1057. (paper)
Other Publications Using This Data