Summary
Primary Aim Findings:
[18F]fluorodeoxyglucose-PET/CT has high NPV for the N0 neck in T2 to T4 HNSCC. The surgical treatment plans on the basis of PET/CT findings may be changed in approximately 22% of this group. These findings suggest that [18F]fluorodeoxyglucose-PET/CT may assist the clinician in deciding on the best therapy for the clinically N0 neck in HNSCC. Well-designed clinical trials should be performed to test the outcome of omitting neck dissection by using PET/CT.
Study Design Summary:
People with newly diagnosed head and neck squamous cell carcinoma being considered for surgical resection, with at least one side of the neck planned for dissection clinically N0, and at risk for occult metastasis (when risk based on clinical data is felt to be greater than 30%). A total of 287 participants were prospectively enrolled from 23 American College of Radiology Imaging Network-qualified institutions. PET/CT was compared with findings at neck dissection.
Two sets of XLS spreadsheets (file set 1 and file set 2) are needed in order to obtain the entire clinical data set for this collection. The file sets are a random sample of ACRIN 6685 participants divided into 2 groups. Group 1/file set 1: a 75% random sample; Group 2: a 25% random sample initially held for testing/validating algorithms trained on the 75% sample.
Acknowledgements
This shared data set was provided in collaboration with the American College of Radiology Core Lab. Many thanks are due to the ACRIN 6685 trial team, and all the patients participating in the study. This study was supported by the American College of Radiology Imaging network (ACRIN), which received funding from the National Cancer Institute through UO1 CA080098, U01 CA180820, U01 CA180794, U01 CA190254, and R50 CA211270 (Muzi), under the American Recovery and Reinvestment ACT of 2009 (ARRA) and UO1 CA079778.
Please see QIN ECOG-ACRIN Data Sharing page for an overview and list of other ECOG-ACRIN data collections available on TCIA.
Data Access
| Data Type | Download all or Query/Filter | License |
|---|---|---|
| Images (DICOM, 138 GB) | (Download requires NBIA Data Retriever) | |
| Clinical Data, file set 1 (XLS, zip, 539 kB) | ||
| Clinical Data, file set 2 (XLS, zip, 479 kB) |
Click the Versions tab for more info about data releases.
Detailed Description
Image Statistics | |
|---|---|
Modalities | CT, PT, MR, NM |
Number of Participants | 260 |
Number of Studies | 681 |
Number of Series | 3358 |
Number of Images | 460,982 |
| Images Size (GB) | 138 |
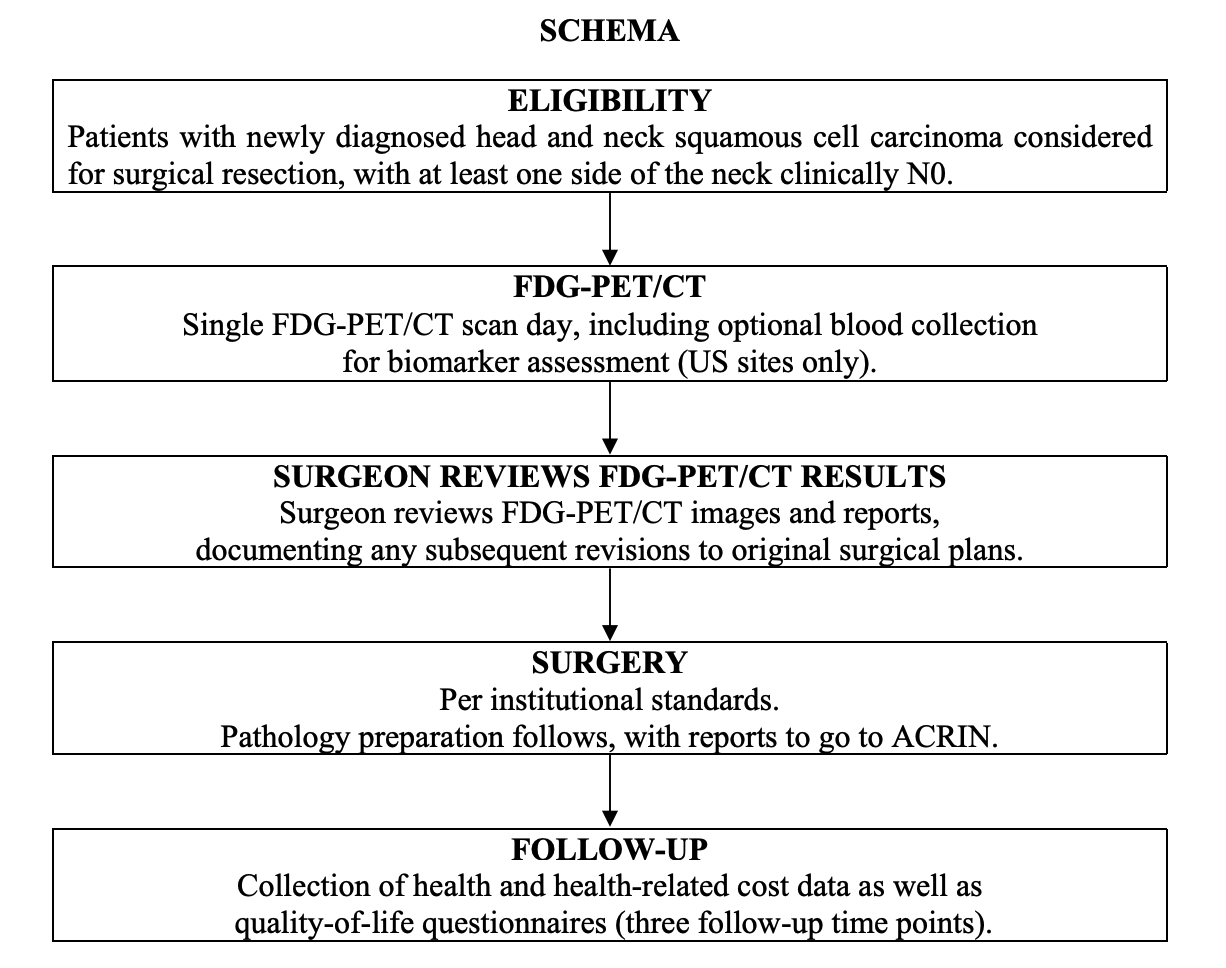
Participant Eligibility and Enrollment:
A total of 287 participants older than 18 years of age were prospectively enrolled from 23 American College of Radiology Imaging Network-qualified institutions. All participants had newly diagnosed, first-time, HNSCC that was being considered for surgical resection. Participants with poorly controlled diabetes (fasting glucose level > 200 mg/dL) were excluded. Eligible participants had HNSCC in the (1) oral cavity; or (2) oropharynx, including the base of the tongue and tonsil; or (3) larynx, including the supraglottis. At least one side of the neck planned for surgical dissection was clinically N0 according to physical examination and preoperative magnetic resonance imaging (MRI) and/or CT evaluation. A clinically N0 neck was defined as being free of palpable lymph nodes and with neck CT and/or MRI neck lymph node sizes of less than 1 cm and 1.5 cm for jugular digastric nodes (IIa), spinal accessory nodes (IIb), or submental-submandibular nodes (Ia and Ib) or showing a lack of central lymph node necrosis in nodes of any size. Participants all received a presurgical FDG-PET/CT scan to which the surgeon was blinded and a contrast-enhanced MRI or CT scan of the neck (all within 4 weeks of surgery). The surgical plans were devised by the local surgeons on the basis of physical examination and CT and/or MRI results, but not PET/CT (pre-PET/CT surgical plan) and thereafter formulated with the PET/CT result (post-PET/CT surgical plan), and both plans were collected prospectively with questionnaires. All participants provided written informed consent and all sites received approval for participation from their local investigational review boards. All data were anonymized to protect the identities of the participants.
Study Overview:
Participants with newly diagnosed head and neck SCC will underwent a FDG-PET/CT scan prior to surgical resection. The surgeon had access to the PET/CT results prior to the surgical procedure. The study collected data on how the inclusion of the PET/CT results impact the determination of extent of disease, disease characterization and prognosis, and the surgical plan originally devised from clinical nodal assessment and CT and/or MRI results.
Participants all received a presurgical FDG-PET/CT scan to which the surgeon was blinded and a contrast-enhanced MRI or CT scan of the neck (all within 4 weeks of surgery). The surgical plans were devised by the local surgeons on the basis of physical examination and CT and/or MRI results, but not PET/CT (pre-PET/CT surgical plan) and thereafter formulated with the PET/CT result (post-PET/CT surgical plan), and both plans were collected prospectively with questionnaires.
Imaging Protocol:
Imaging with FDG-PET/CT was performed with a dedicated head and neck (H&N) PET/CT using two bed positions from the orbits to the upper thorax (top of aortic arch) with arms down and with images acquisitions at 6 minutes per bed position. Of the 248 eligible participants, 54 underwent H&N scans only, 30 underwent whole-body (WB) scans only, and six underwent neither. Imaging from the orbits or chest through the upper thighs was also obtained. See the Data Supplement for detailed methods of imaging, image analysis, surgery methods, and pathology methods.
Results:PET/CT scans and pathology findings were available for 270 N0 neck sides from 212 participants. For visual assessment, the NPV specific to the clinical-N0 sides was 0.868 (95% CI, 0.803 to 0.925). For dichotomized maximum standardized uptake value, the NPVs specific to the nodal basins were 0.940 (95% CI, 0.928 to 0.952) and 0.937 (95% CI, 0.925 to 0.949) at prespecified cutoffs of 2.5 and 3.5, respectively. The optimal cutoff maximum standardized uptake value was determined to be 1.8, with an NPV of 0.942 (95% CI, 0.930 to 0.953). The PET/CT-informed surgical treatment plan was changed in 51 of 237 participants (22%) compared with the PET/CT-blinded surgical plan. In 34 participants (14%), this led to planned dissection of additional nodal levels. In 12 participants (5%), this led to fewer planned dissected nodal levels. Negative PET/CT scans in N0 necks was true negative in 87% and false negative in 13%.
Conclusion:
PET/CT has a high NPV for N0 HNSCC. SUV analysis is superior to visual assessment with regard to NPV. There is a high degree of reader agreement for PET/CT of the patient with an N0 neck. These findings suggest that FDG-PET/CT may assist the clinician in deciding on the best therapy for the clinically N0 neck in HNSCC.
Date Offsets:
All dates, like the visit date, are protected by presenting just the year; however, dates are also listed as offset days from the base date. The offset dates are used as a means of protecting patient information provided by the local sites in the original data, while allowing users to determine intervals between events. The standard DICOM date tags (i.e. birth dates, imaging study dates, etc.) have been de-identified so that all patients have a baseline study date of January 1, 1960. This falsified date represents the day patients were entered into trial database. The number of days between a subject’s longitudinal imaging studies are accurately preserved. A patient with a study performed on January 4, 1960 means the images were collected 3 days after the base date. For convenience, this calculation has been performed for all scans with the results inserted in DICOM tag (0012,0050) Clinical Trial Time Point ID. This means an imaging study that took place on January 4, 1960 would contain a value of "3" in tag (0012,0050).
Overview of Clinical Data:
The basic data flow for legacy ACRIN multi-center clinical trials was that all clinical information provided by the local imaging sites were contained in a series of forms. The form data submitted by local investigators to ACRIN during and after the trial, were manually encoded into the ACRIN CTMS (Clinical Trial Management System), and were cross-checked for accuracy by ECOG-ACRIN personnel. These ACRIN 6685 forms (see the ACRIN 6685 Data Forms page), filled out by the local sites, deliver information on imaging, clinical management of the patient and pathology/outcome variables, like dates of progression and survival, along with other critical information. The image data was initially anonymized while uploading from the local sites through TRIAD software and archived in a DICOM database at ACRIN.
After the trial accrual had ended, the clinical data was sent to the Brown statistical center, that is funded by NCI to provide support for ECOG-ACRIN clinical trials, specifically for analysis of the primary and sometimes secondary aims of the trial. The statisticians at Brown strip all the actual dates, names and other PHI from the CTMS data and create a .csv file for each form that has selected information useful for analysis of the trial data. A Form Booklet pdf file detailing all the forms used in the study accompanies the .csv data files. Additionally, the accompanying Data Dictionary file lists each element for each form that has been selected for data retention along with a description of each form element.
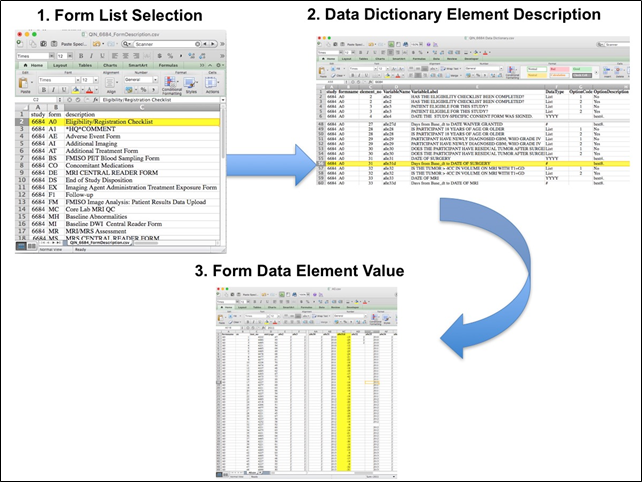
Extracting clinical (non-imaging) data example:
Beginning with the Form Booklet_6685.pdf file, select the form with the desired information needed, such as form A0.csv the patient Eligibility/Registration form. Next, using the Data Dictionary.xls file, find the form elements listed for A0 (eg., A0exx, where xx is the form element number). The file lists the form number, variable name, its description or label, the type of data, and, when applicable, the option codes and corresponding text values (option code:description pairs like 1=’No’, 2=’Yes’; or 1=’Baseline’, 2=’Post treatment’) for each data element available from the form. In the example in Figure 2, the A0 form element 31d (A0e31d) reports the days between the base date and the day of surgery for the patient. In the corresponding A0.csv file column G lists the days between the base date and surgery for each patient.
In this example of extracting clinical data, the first step is to 1) find the form from the form list, 2) Find the desired element and description in the Data Dictionary and finally 3) extract the values from the .csv data file.
The procedure above is basically how the statisticians organized the selected data for export, but the structure of the data dictionaries and individual forms are different for each clinical trial.
Citations & Data Usage Policy
Users must abide by the TCIA Data Usage Policy and Restrictions. Attribution should include references to the following citations:
Data Citation
Kinahan, P., Muzi, M., Bialecki, B., & Coombs, L. (2019). Data from the ACRIN 6685 Trial HNSCC-FDG-PET/CT [Data set]. TCIA. https://doi.org/10.7937/K9/TCIA.2016.JQEJZZNG
Publication Citation
Lowe, V. J., Duan, F., Subramaniam, R. M., Sicks, J. D., Romanoff, J., Bartel, T., Yu, J. Q. (Michael), Nussenbaum, B., Richmon, J., Arnold, C. D., Cognetti, D., & Stack, B. C., Jr. (2019). Multicenter Trial of [18F]fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Staging of Head and Neck Cancer and Negative Predictive Value and Surgical Impact in the N0 Neck: Results From ACRIN 6685. Journal of Clinical Oncology, 37(20), 1704–1712. https://doi.org/10.1200/jco.18.01182
TCIA Citation
Clark, K., Vendt, B., Smith, K., Freymann, J., Kirby, J., Koppel, P., Moore, S., Phillips, S., Maffitt, D., Pringle, M., Tarbox, L., & Prior, F. (2013). The Cancer Imaging Archive (TCIA): Maintaining and Operating a Public Information Repository. Journal of Digital Imaging, 26(6), 1045–1057. https://doi.org/10.1007/s10278-013-9622-7
Other Publications Using This Data
TCIA maintains a list of publications which leverage TCIA data. If you have a manuscript you'd like to add please contact TCIA's Helpdesk.
Version 2 (Current): Updated 2021/11/23
| Data Type | Download all or Query/Filter |
|---|---|
| Images (DICOM, 138 GB) | (Requires NBIA Data Retriever.) |
| Clinical Data, file set 1 (XLS, zip) | |
| Clinical Data, file set 2 (XLS, zip) |
Note: Corrected Body Part Examined to appropriate DICOM code string, study description modified for same data to remove extraneous/unclear text
Version 1: Updated 2020/03/13
| Data Type | Download all or Query/Filter |
|---|---|
| Images (DICOM, 138 GB) | (Requires NBIA Data Retriever.) |
| Clinical Data, file set 1 (XLS, zip) | |
| Clinical Data, file set 2 (XLS, zip) |