Summary
RTOG 0625/ACRIN 6677 is a multicenter, randomized, phase II trial of bevacizumab with irinotecan or temozolomide in recurrent glioblastoma (GBM). This study investigated whether early posttreatment progression on FLAIR or postcontrast MRI assessed by central reading predicts overall survival (OS). Full details can be found in the primary publication about this trial and at https://www.acrin.org/6677_protocol.aspx. Of 123 enrolled patients, 107 had baseline and at least 1 posttreatment MRI. Two central neuroradiologists serially measured bidimensional (2D) and volumetric (3D) enhancement on postcontrast T1-weighted images and volume of FLAIR hyperintensity. Progression status on all posttreatment MRIs was determined using Macdonald and RANO imaging threshold criteria, with a third neuroradiologist adjudicating discrepancies of both progression occurrence and timing. For each MRI pulse sequence, Kaplan-Meier survival estimates and log-rank test were used to compare OS between cases with or without radiologic progression. Radiologic progression occurred after 2 chemotherapy cycles (8 weeks) in 9 of 97 (9%), 9 of 73 (12%), and 11 of 98 (11%) 2D-T1, 3D-T1, and FLAIR cases, respectively, and 34 of 80 (43%), 21 of 58 (36%), and 37 of 79 (47%) corresponding cases after 4 cycles (16 weeks). Median OS among patients progressing at 8 or 16 weeks was significantly less than that among nonprogressors, as determined on 2D-T1 (114 vs 278 days and 214 vs 426 days, respectively; P < .0001 for both) and 3D-T1 (117 vs 306 days [P < .0001] and 223 vs 448 days [P = .0003], respectively) but not on FLAIR (201 vs 276 days [P = .38] and 303 vs 321 days [P = .13], respectively). Early progression on 2D-T1 and 3D-T1, but not FLAIR MRI, after 8 and 16 weeks of anti-vascular endothelial growth factor therapy has highly significant prognostic value for OS in recurrent GBM. Acknowledgements This shared data set was provided in collaboration with the American College of Radiology Core Lab. Many thanks are due to the ACRIN 6677 trial team, and all the patients participating in the study. This study was supported by ACRIN, which received funding from the National Cancer Institute through UO1 CA080098, under the American Recovery and Reinvestment ACT of 2009 (ARRA) and UO1 CA079778.METHODS:
RESULTS:
CONCLUSION:
Data Access
This is a limited access data set and is only available to members of NCI's Quantitative Imaging Network (QIN) with an anticipated public release of 09/05/2020. If you are a member of the QIN and would like to request access, please submit a CCP proposal to the QIN Coordinating Committee. Once access is granted, click the Download button to save a ".tcia" manifest file to your computer, which you must open with the NBIA Data Retriever. Click the Search button to open our Data Portal, where you can browse the data collection and/or download a subset of its contents.
Click the Versions tab for more info about data releases.
Detailed Description
Collection Statistics | |
|---|---|
Modalities | MR, CT |
Number of Patients | 123 |
Number of Studies | 566 |
Number of Series | 7629 |
Number of Images | 717,070 |
| Image Size (GB) | 86.9 |
Study Accrual:
Of the 123 patients enrolled on the study, 107 had baseline and at least 1 post-treatment MRI and were evaluable for the primary aims.
Imaging Protocol:
The MR imaging protocol is divided into two sections: Standard and Advanced. The Standard protocol acquires a pre-contrast T1-weighted, a T2-weighted, a FLAIR and a diffusion-weighted imaging series all in the axial plane. After intravenous injection of 0.1 mmol/kg of standard gadolinium-based agent, axial 2D spin-echo (2D-T1) and 3D volumetric (3D-T1) T1-weighted (post-Gd) images were acquired.
The advanced scheme acquires the following series: a T1 mapping sequence with flip angles at 2°, 5°, 10°, 15°, 25°, a dynamic contrast-enhanced T1, a dynamic susceptibility contrast diffusion weighted series and/or a MR spectroscopy 2D CSI PRESS sequence. Complete MRI parameters for this protocol are listed on the ACRIN website http://www.acrin.org/6677_protocol.aspx, see the Imaging Transmittal Worksheet and Parameters in the Imaging Materials link.
Image Analysis:
Two central neuroradiologists serially measured bi-dimensional (2D) and volumetric (3D) enhancement on post-contrast T1-weighted images and volume of FLAIR hyperintensity on 107 evaluable patients. For the MR spectroscopy analysis, 13 patients who had baseline and subsequent MRS were analyzed. Spectra from the enhancing tumor and peri-tumoral regions were defined on the post-contrast T1-weighted images. Changes in the concentration ratios of n-acetylaspartate/creatine (NAA/Cr), choline-containing compounds (Cho)/Cr, and NAA/Cho were quantified in comparison with pretreatment values.
Outcomes:
Outcome, like progression status, on all post-treatment MRIs was determined using Macdonald and RANO imaging threshold criteria, with a third neuroradiologist adjudicating discrepancies of both progression occurrence and timing. For each MRI pulse sequence, Kaplan-Meier survival estimates and log-rank test were used to compare OS between cases with or without radiologic progression. Results indicated that early progression on 2D-T1 and 3D-T1, but not FLAIR MRI, after 8 and 16 weeks of anti-vascular endothelial growth factor therapy has highly significant prognostic value for OS in recurrent GBM. For MR spectroscopy, NAA/Cho levels increased and Cho/Cr levels decreased within enhancing tumor at 2 weeks relative to pretreatment levels. Decreased Cho/Cr and increased NAA/Cr and NAA/Cho in tumor periphery at 16 weeks posttreatment were associated with both 6-month progression-free survival and 1-year survival.
Date Offsets:
All dates, like the visit date, are protected by presenting just the year; however, dates are also listed as offset days from the base date. The offset dates are used as a means of protecting patient information provided by the local sites in the original data, while allowing users to determine intervals between events. The standard DICOM date tags (i.e. birth dates, imaging study dates, etc.) have been de-identified so that all patients have a baseline study date of January 1, 1960. This falsified date represents the day patients were entered into trial database. The number of days between a subject’s longitudinal imaging studies are accurately preserved. A patient with a study performed on January 4, 1960 means the images were collected 3 days after the base date. For convenience, this calculation has been performed for all scans with the results inserted in DICOM tag (0012,0050) Clinical Trial Time Point ID. This means an imaging study that took place on January 4, 1960 would contain a value of "3" in tag (0012,0050).
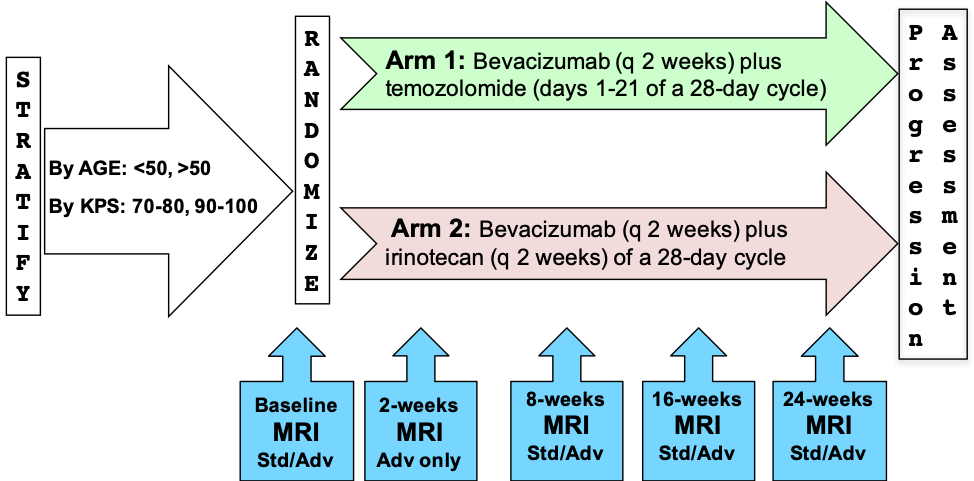
Schema
Please refer to the imaging protocol for the definitions of standard (Std) and advanced (Adv) MR imaging. Assessment of progression was determined by Macdonald and RANO criteria.
Citations & Data Usage Policy
This is a limited access data set and is only available to members of NCI's Quantitative Imaging Network (QIN) until 09/05/2020. If you are a member of the QIN and would like to request access, please submit a CCP proposal to the QIN Coordinating Committee. Upon receiving access you may only use it for the purposes outlined in your proposal. You are not allowed to redistribute the data or use it for other purposes. See TCIA's Data Usage Policies and Restrictions for additional details. Questions may be directed to help@cancerimagingarchive.net.
Please be sure to include the following citations in your work if you use this data set:
Data Citation
Kinahan, P., Muzi, M., Bialecki, B., Herman, B., & Coombs, L. (2019). Data from ACRIN-DSC-MR-Brain [Data set]. The Cancer Imaging Archive. DOI: https://doi.org/10.7937/tcia.2019.zr1pjf4i.
Publication Citation
Boxerman JL, Zhang Z, Safriel Y, et al. Early post-bevacizumab progression on contrast-enhanced MRI as a prognostic marker for overall survival in recurrent glioblastoma: results from the ACRIN 6677/RTOG 0625 Central Reader Study. Neuro Oncol. 15:945-954. doi: 910.1093/neuonc/not1049. Epub 2013 Jun 1019.; 2013.
Ratai EM, Zhang Z, Snyder BS, et al. Magnetic resonance spectroscopy as an early indicator of response to anti-angiogenic therapy in patients with recurrent glioblastoma: RTOG 0625/ACRIN 6677. Neuro Oncol. 15:936-944. doi: 910.1093/neuonc/not1044. Epub 2013 May 1093.; 2013.
Ellingson BM, Kim E, Woodworth DC, et al. Diffusion MRI quality control and functional diffusion map results in ACRIN 6677/RTOG 0625: a multicenter, randomized, phase II trial of bevacizumab and chemotherapy in recurrent glioblastoma. Int J Oncol. 46:1883-1892. doi: 1810.3892/ijo.2015.2891. Epub 2015 Feb 1811.; 2015.
Schmainda KM, Zhang Z, Prah M, et al. Dynamic susceptibility contrast MRI measures of relative cerebral blood volume as a prognostic marker for overall survival in recurrent glioblastoma: results from the ACRIN 6677/RTOG 0625 multicenter trial. Neuro Oncol. 17:1148-1156. doi: 1110.1093/neuonc/nou1364. Epub 2015 Feb 1142.; 2015.
Barboriak DP, Zhang Z, Desai P, et al. Interreader Variability of Dynamic Contrast-enhanced MRI of Recurrent Glioblastoma: The Multicenter ACRIN 6677/RTOG 0625 Study. Radiology. 290:467-476. doi: 410.1148/radiol.2019181296. Epub 2019182018 Nov 2019181227.; 2019.
Schmainda KM, Prah MA, Zhang Z, et al. Quantitative Delta T1 (dT1) as a Replacement for Adjudicated Central Reader Analysis of Contrast-Enhancing Tumor Burden: A Subanalysis of the American College of Radiology Imaging Network 6677/Radiation Therapy Oncology Group 0625 Multicenter Brain Tumor Trial. AJNR Am J Neuroradiol. 40:1132-1139. doi: 1110.3174/ajnr.A6110. Epub 2019 Jun 1127.; 2019.
TCIA Citation
Clark K, Vendt B, Smith K, Freymann J, Kirby J, Koppel P, Moore S, Phillips S, Maffitt D, Pringle M, Tarbox L, Prior F. The Cancer Imaging Archive (TCIA): Maintaining and Operating a Public Information Repository, Journal of Digital Imaging, Volume 26, Number 6, December, 2013, pp 1045-1057. DOI: 10.1007/s10278-013-9622-7
Other Publications Using This Data
TCIA maintains a list of publications which leverage our data. At this time we are not aware of any publications based on this data. If you have a publication you'd like to add please contact the TCIA Helpdesk.